Essay On Water Molecules. A water molecule is made of two hydrogen atoms and one oxygen atom. Oxygen and Hydrogen are gases but when combined form into a liquid compound; water. The hydrogen atoms make an angle of around degrees with the oxygen atom. The oxygen atom dominates an end of the molecule while the hydrogen atoms dominate the other Apr 21, · Water is a chemical compound with the chemical formula H 2O. A water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and pressure, but it often co-exists on Earth with its solid state, ice, and gaseous state, steam (water vapor) Jun 19, · Order custom essay Water Molecule with free plagiarism report. GET ORIGINAL PAPER. The oxygen atom also called the "apex of the water molecule" bears a slight electronegative charge while hydrogen possesses a more positive one (Kirk ). Because of the opposite charges attract, the water molecules are drawn blogger.comted Reading Time: 4 mins
Water Molecule - blogger.com
This also traps just enough warmth to keep marine animals alive during the winter. The process of turning water into steam is a different story. Because it requires the breaking of water's hydrogen bonds, this process takes far more energy than it does to turn water into ice, water molecule essay. The extra energy that is used in. its boiling point would be ºC rather than ºC. Water can also be used as a solvent because of it polarity, water molecule essay. Many things will dissolve in it, water molecule essay, and more reactions take place while in solution with water.
Often in organisms substances must be in solution and water is the solvent. Plants can only obtain mineral salts in solution so require water to live. Without the hydrogen bonds, water too would be a gas at RTP and would have a boiling point of °C. Because it is polar, water also has uses as a solvent, water molecule essay. Other polar substance are dissolved by water as the electrostatic attractions between the water molecules and the ions are greater that the attraction between the anion and cation.
The water molecules surround the ions and thus the ions become water molecule essay. Polar substances that dissolve in water are said to be hydrophilic or "water-loving".
Latent heat of fusion. Water also requires a lot of heat to change state from a solid to a liquid, and must loose a lot of heat to change state from a liquid to a solid. This means it is difficult to freeze water, so ice crystals are less likely to form inside cells.
Water is unique in that the solid state ice is less dense that the liquid state, so ice floats on water. As water cools toward 0°C, the water molecules slow down to form the maximum number of hydrogen bonds. As this process continues, water molecules must give enough space for all four hydrogen bonds to fit, causing the water to expand as it freezes to form ice. Ice is less dense than the liquid form and therefore floats, a property very significant to the survival of fish as this layer of ice insulates the liquid water below to prevent the whole lake or river to freeze.
Otherwise, this could be fatal for many organisms in the aquatic food web. Aquatic plants can survive in deep waters due to the fact th middle of paper er as sweat, the liquid gets turned into a vapour causing water molecule essay to water molecule essay down and control our body temperature a homeostasis process.
Therefore, the heat is removed and released with the water vapour into the air. Conclusion In summary, the existence of water in all three states of matter on earth is vital to all living organisms.
This incomparable molecule acquires its unique properties because of the suitable atmospheric conditions on earth and the strong intermolecular hydrogen bonds binding the molecules together. The life supporting properties of water include its widespread dissolving power, unusual change in density among the states of matter, cohesive and adhesive nature, high specific heat, and high heat of vaporization. Without water water molecule essay its properties, the diverse range of life on earth would not exist.
How Water Is Related To Chemistry Water is a polar solvent, its molecule is covalently bonded that makes up for an unequal sharing of the electrons resulting in partially positive and partially negative water molecule. Organic molecules like Ethane and many other molecules are non-polar, that is they neither have a positive nor a negative end. As a result, water molecule essay individual molecules within the water are greatly interconnected because of the presence of weak hydrogen bonds.
Water acts as a universal solvent. All the living things are made up of entities, called atoms and molecules, and these entities are inside aqueous solutions that is the solutions containing elements dissolvable in water. When water freezes, the water molecules are no longer free to move and the hydrogen bonds are permanently formed.
Ice floats because ice is less dense than water. This is because in the solid state the molecules are less closely packed than in liquid water. Fish can water molecule essay in frozen lakes because the ice on the surface insulates the water from further freezing. Water is also good as a lubricant. The Biological Importance of Water Water is a substance whose molecule is made from two hydrogen atoms and one oxygen atom H20 and that is in a liquid state at room temperature.
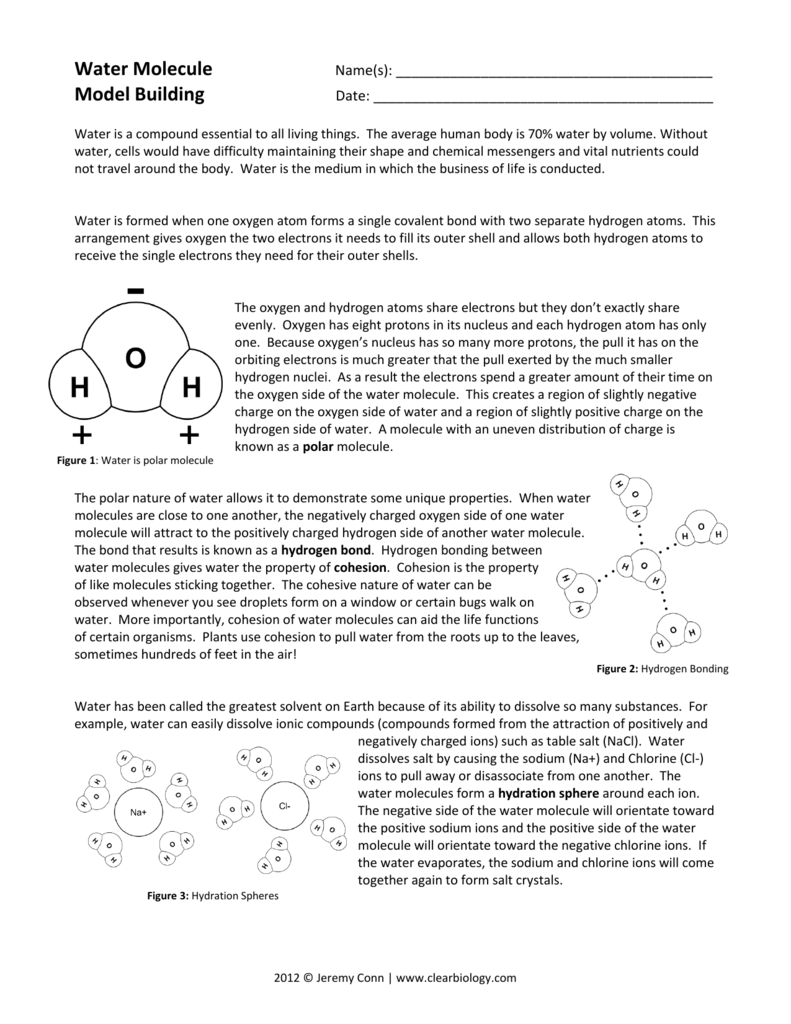
This is covalent bonding, where two hydrogen atoms share their electron with one oxygen atom. As the oxygen atom has more positively charged protons than the hydrogen is has pulls on the shared electrons more strongly than the hydrogen water molecule essay. The oxygen atom therefore has a slightly negative charge while the hydrogen a slightly positive charge.
Therefore because the hydrogen and oxygen atoms are different in size and electronegativity the water molecule is non-linear and dipolar. Oxygen has greater electronegativity so has a greater pull on the electron causing the electrons to move away slightly from the hydrogen atoms. These forces of attraction are called hydrogen bonding, which gives water its unique properties.
The hydrogen bonding makes water molecules difficult to separate, water molecule essay. A greater amount of energy is needed to break the hydrogen bonds to convert water into a gas than is needed for similar compounds such as hydrogen sulphide H2S.
Home Page The Chemical Properties of Water. The Chemical Properties of Water Satisfactory Essays. Open Document. Essay Sample Check Writing Quality. Water is the most important substance in our evolution and our daily lives. Without water, life as we know it would not have been possible. This essay will examine the water molecule in order to ascertain how it brought about Earth's thriving ecosystem and how important water molecule essay is to us today, water molecule essay.
Each water molecule consists of one oxygen atom and two hydrogen atoms. The oxygen atom or the apex of the water molecule bears a slight electronegative charge while hydrogen possesses a more positive one, water molecule essay. Because opposite charges attract, the water molecules are drawn together.
When an oxygen atom is linked to a neighboring molecule's hydrogen atom, a bond called a hydrogen bond is formed. In an ice crystal the hydrogen bonds to give the shape of the crystal so that the grid of molecules surrounds relatively to large spaces. In a liquid form, water has no such spaces; so ice is less dense and will float on liquid water. If not for this, great bodies of water would freeze from the bottom up without the water molecule essay of a top layer of ice and all life in the water would die.
The water molecule is a very small one but because of its unique properties it behaves like a larger one. The bonds between water molecules are so strong that water resists changes in its state Solid, liquid, water molecule essay, gas ; thus water has a higher melting point and a higher boiling point than another molecule of similar size.
If water followed the example of other molecules its size it would have a boiling point of øC and a freezing point of øC4. This would mean water molecule essay, on Earth, water would be a gas all of the time and life would not be possible. When heat is applied to solid water, some hydrogen bonds get so much kinetic energy that they break and the ice melts. Liquid water does not necessarily have all four hydrogen bonds present at all times but it must retain some of them.
All plant life on Earth benefits from the ability of water to make a hydrogen bond with another substance of similar electronegative charge. Cellulose, the substance that makes up cell walls and paper products, is a hydrophilic substance "water-loving". It interacts with water but, unlike other hydrophilic substances, it will not dissolve in it, water molecule essay. Cellulose can form strong hydrogen bonds with water molecules.
This explains why a paper towel will "wick" water upwards when it comes in contact with it, water molecule essay. Get Access. Satisfactory Essays, water molecule essay. The Chemical Properties Of Water Words 3 Pages. The Chemical Properties Of Water.
Read More. The Role Of Water In Living Organisms Words 2 Pages. The Role Of Water In Living Organisms. The Role of Water in Living Organisms Words 2 Pages. The Role of Water in Living Organisms. Investigating Water Words 1 Pages. Investigating Water. Good Essays. The Role of Water in the Lives of Organisms Words 2 Pages. The Role of Water in the Lives of Organisms, water molecule essay. Importance Of Water To Life Words 4 Pages, water molecule essay.
Importance Of Water To Life. How Water Is Related To Chemistry Words 2 Pages 1 Works Cited. How Water Is Related To Chemistry.
The Biological Importance of Water Words 1 Pages. The Biological Importance of Water. The Biological Importance of Water Words 2 Pages. The Properties of Water and It's Significance to Living Processes Words 2 Pages. The Properties of Water and It's Significance to Living Processes. Related Topics. Freeze Freezing Liquid Molecules Water Oxygen.
Water Has Memory! Dr. Masaru Emoto's Water Experiment!
, time: 2:44Essay On Water Molecules - Words | Cram
Jun 19, · Order custom essay Water Molecule with free plagiarism report. GET ORIGINAL PAPER. The oxygen atom also called the "apex of the water molecule" bears a slight electronegative charge while hydrogen possesses a more positive one (Kirk ). Because of the opposite charges attract, the water molecules are drawn blogger.comted Reading Time: 4 mins Essay On Water Molecules. A water molecule is made of two hydrogen atoms and one oxygen atom. Oxygen and Hydrogen are gases but when combined form into a liquid compound; water. The hydrogen atoms make an angle of around degrees with the oxygen atom. The oxygen atom dominates an end of the molecule while the hydrogen atoms dominate the other Apr 21, · Water is a chemical compound with the chemical formula H 2O. A water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and pressure, but it often co-exists on Earth with its solid state, ice, and gaseous state, steam (water vapor)
No comments:
Post a Comment